If realized using solar energy or other renewable energy, water splitting could be a promising way of sustainably producing hydrogen (H2) on a large-scale. Most photoelectrochemical water splitting systems proposed so far, however, have been found to be either inefficient, unstable, or difficult to implement on a large-scale.
Researchers at Ulsan National Institute of Science and Technology (UNIST) recently set out to develop a scalable and efficient photoelectrochemical (PEC) system to produce green hydrogen. Their proposed system, outlined in Nature Energy, is based on an innovative formamidinium lead triiodide (FAPbI3) perovskite-based photoanode, encapsulated by an Ni foil/NiFeOOH electrocatalyst.
“Our group has thoroughly studied the challenges associated with practical solar hydrogen production,” Jae Sung Lee, Professor of Energy & Chemical Engineering at UNIST and co-author of the paper, told Tech Xplore. “As summarized in our most recent review paper, minimum 10% of solar-to-hydrogen (STH) efficiency is required to develop viable practical PEC system, for which selecting an efficient material is the first criteria.”
So far, most attempts at realizing photoelectrochemical hydrogen production employed intrinsically stable metal oxides as the photoelectrode materials of PEC cells. These systems, however, have yielded efficiencies far below those necessary for their practical application.
Some researchers have thus been exploring the potential of photoelectrodes based on photovoltaic (PV) grade materials, such as silicon, perovskites, chalcogenides and III-V material classes. While these materials are known for their remarkable efficiencies, they can sometimes be expensive and unstable, especially if placed in water, as they would be if introduced in PEC water splitting cells.
“Unlike other PV grade materials, metal-halide perovskites (MHP) have unique characteristics of high efficiency but low cost and could become an alternative photoelectrode material if their stability issue is properly addressed,” Lee said. “The MHP materials have excellent optoelectronic properties and tunable bandgap which are desired to provide necessary photocurrent and photovoltage to split water and produce oxygen and hydrogen in a single PEC cell.”
To devise effective photoelectrodes based on MHPs, the researchers first had to tackle a crucial challenge, namely that of maintaining their stability in humid conditions and under UV light. To achieve this, they tried to stabilize them using metal-encapsulation or metal-protection techniques and by adopting the UV-stable FAPbI3 perovskite.
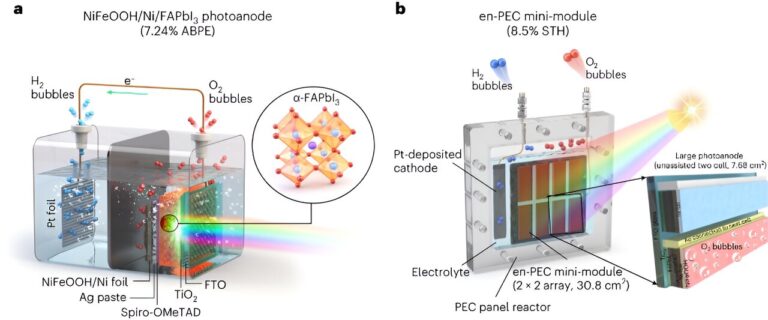
“Another challenge for practical applications is the scalability, or in other words, to maintain the high efficiency of laboratory cells (which are < 1 cm2) in practical large-scale implementations (1 m2),” Lee said. “For our study, we selected the most advanced MHP material in efficiency and stability (FAPbI3) and encapsulated it with a thick nickel foil (30 mm) deposited with an NiFeOOH catalyst to protect MHP in water and promote oxygen evolution reaction for water splitting,”
The researchers firstly created a small-scale version of their proposed system, based on a photoelectrode below 1cm2 in size. In initial tests, this laboratory-scale system achieved a 9.89% STH efficiency and a long-term stability.
“We then scaled up this small-area device to practical large area PEC system using module-based design,” Lee said. “We did this by selecting a 7.68 cm2 device as the basic mini-module and repeated it horizontally and vertically to fabricate a large-size device.”
Remarkably, Lee and his colleagues found that the upscaling of their system only resulted in a minimal loss of efficiency. In addition, the upscaled system maintained its long-term stability, suggesting that their design is highly scalable.
“Our all-perovskite PEC system is composed of FAPbI3 photoanode, which is a MHP thin film protected using nickel metal foil as an encapsulation layer and NiFeOOH as a catalyst layer on it,” Lee said.
“We optimized this photoanode using different metal foils and studied the in-depth catalyst-electrolyte interactions. This photoanode was connected in parallel to another MHP thin film as PV in a single reactor to generate enough voltage (~2 V) to split water molecules into O2 and H2 gases. In a large scale system, both components (photoanode and PV) are integrated in single PEC device to simplify the total system by a modular design.”
The researchers’ mini-module is essentially made up of a photoelectrode and a PV unit cell, arranged in a 4 x 4 array. Their system integrates multiple components in a single PEC device to eliminate the need for additional PV components.
This unique design reduces their system’s complexity and lowers its fabrication costs. Lee and his colleagues demonstrated that their system retains a good performance even when deployed in a larger scale, which could facilitate its future real-world deployment.
“The short-term demonstration of our scalable system will lead toward practical application of PEC technology for green hydrogen production in outdoor conditions,” Lee added. “We also plan to further improve the efficiency and stability of the PEC system by integration of photoelectrodes and selecting more efficient and durable catalyst. We look [forward to] the opportunity to demonstrate a pilot scale solar hydrogen production system under natural sunlight using our technology.”
More information:
Dharmesh Hansora et al, All-perovskite-based unassisted photoelectrochemical water splitting system for efficient, stable and scalable solar hydrogen production, Nature Energy (2024). DOI: 10.1038/s41560-023-01438-x
© 2024 Science X Network
Read the full article here